Calculate the volume of CO₂ gas in L collected over water at 250 C when 251 g of calcium carbonate is added to excess hydrochloric acid if the total pressure is 911 mm Hg. ZnCO3s ZnOs CO2g Owing to the conditions under which the reaction occurs it is not possible to measure the enthalpy change directly.

On Heating Calcium Carbonate Gets Converted Into Calcium Oxide And Carbon Dioxide Youtube
I Name the compound X and give its chemical formula.

. It melts without decomposition at 852 o C. C 2 H 6 O 2 2CO 2 3H 2 O. Ii Write a balanced chemical equation to represent the decomposition of X.
Given the balanced equation LiOH KCl - LiCl KOH what is the theoretical yield of lithium chloride if you start this reaction with 200 grams. 5 pts Given the following balanced net ionic equation. This thorough mixing is a chemical reaction.
It then decomposes to ferric oxide Fe2O3 sulphur dioxide SO2 and sulphur trioxide SO3. Now to balance the atoms in carbon multiply CO 2 molecules by 2. 3 16 A compound X of sodium is used as an antacid and it decomposes on strong heating.
Thus complete conversion of the starting materials to product requires equimolar amounts of acid and alcohol. Mn Cr2 aq Mn2 aq Cr s A. Thus after removing the.
Calcium oxide is called lime or quick lime. When ferric hydroxide is heated it decomposes into ferric oxide and water 2FeOH 3 s underrightarrow triangle Fe 2 O 3 s 3H 2 Ol Thermal Decomposition. Related CBSE Class 10 Science Syllabus 2021 Session.
Anhydrous sodium carbonate is stable towards heat. We conclude that they thoroughly mix with each other and form a new substance. Magnesium hydroxide decomposes on heating forming magnesium oxide and water.
On which chemical law balancing of chemical equation is based. MgOH 2 s MgO s H 2 O g When a sample of MgOH 2 was heated in a crucible over a Bunsen burner flame the weight of the crucible and its contents went from 4378 g to 4256 g. For example calcium carbonate decomposes into calcium oxide and carbon dioxide.
CaCO₃s CaOs CO₂g In some compounds the energy needed for decomposition is so small that it can be supplied by a minor shock such as a physical impact. There are 7 O-atoms on RHS. Iii Write a balanced chemical equation to represent the above reaction.
CeCaCO_3 left s right rightarrow ceCaO left s right ceCO_2 left g right Metal hydroxides decompose on heating to yield metal oxides and water. Upon heating calcium carbonate decomposes to produce calcium oxide and carbon dioxide. A oxidation reaction is the loss of electrons during a reaction by question_answer.
When heated leadII nitrate decomposes to form leadII oxide nitrogen dioxide and molecular oxygen. Balancing of a chemical equation is based on the law of conservation of mass. The balanced equation for n-butyl acetate synthesis equation 2 shows that the reaction requires the same number of moles of acetic acid as of n-butyl alcohol.
Video Explanation of Lesson Chemical. Decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is an important decomposition reaction used in various industries. Water vapor reacts with.
Gaseous butane C 4 H 10 reacts with diatomic oxygen gas to yield gaseous carbon dioxide and water vapor. 3 On strong heating zinc carbonate decomposes to zinc oxide and carbon dioxide. What would happen if the amounts taken for a particular reaction were not.
C 2 H 6 72 O 2 2CO 2 3H 2 O x 2. Name the term used for the solution of the reactants or products when dissolved in water. For example calcium carbonate decomposes into calcium oxide and carbon dioxide.
Chemical Properties of Sodium Carbonate Na 2 CO 3 1. How many grams of MgOH 2 were initially present. The decomposition of a substance on heating is known as Thermal Decomposition.
The cell is made up of a steel tank with heat-resistant bricks lining it. Na 2 CO 3 s 2H 2 Ol H 2 CO 3 aq 2Na aq 2OH aq. Calcium carbonate decomposes on heating to give calcium oxide and carbon dioxide.
To make 7 O-atoms at LHS we have to write 72 before O 2 but we can use only whole number to balance the equation so we write 72before O 2 and multiply the whole equation by 2. Aqueous solutions of sodium carbonate are mildly alkaline due to hydrolysis which releases OH aq ions. Solid calcium carbonate is heated and decomposes to solid calcium oxide and carbon dioxide gas.
For example if calcium carbonate is strongly heated it decomposes into calcium oxide and carbon dioxide. During the hypochlorite oxidation of. A metal carbonate decomposes into a metal oxide and carbon dioxide gas.
Aqueous solutions of magnesium chloride and sodium hydroxide react to produce solid magnesium hydroxide and aqueous sodium chloride. The vapor pressure of. An indirect method uses the enthalpy changes when zinc carbonate and zinc oxide are neutralized with hydrochloric acid.
Ferric oxide is a solid while SO2 and SO3 are gases. On heating it turns into tea. In the centre of the cell a circular graphite anode is installed which is encircled by a cylindrical iron cathode.
When a mixture of aluminum and ironII oxide is heated metallic iron and aluminum. A metal carbonate decomposes into a metal oxide and carbon dioxide gas. The complete combustion of isopropyl alcohol C3H7OH produces carbon dioxide and water vapor.
CeCaCO_3 left s right rightarrow ceCaO left s right ceCO_2 left g right Metal hydroxides decompose on heating to yield metal oxides and water. Write a balanced chemical equation for each reaction. It includes employing iron as a cathode and graphite as an anode to electrolyze fused sodium chloride containing calcium chloride and potassium fluoride at roughly 600 degrees Celsius.
In the hypochlorite oxidation of cyclohexanol to cyclohexanone what purpose does the acetie-acid A. When solid calcium carbonate is reacted with aqueous hydrochloric acid the products of the reaction include aqueous calcium chloride liquid water and gaseous carbon dioxide. Calcium carbonate Calcium oxide Carbon dioxide.
What is the percent yield of CO_2 if 975 g CO_2 is collected. Oxidation and reduction reaction. In the reaction of hydrogen.
Likewise if you add a spoon of curd to lukewarm milk and keep it aside for few hours then the whole milk turns into curd.
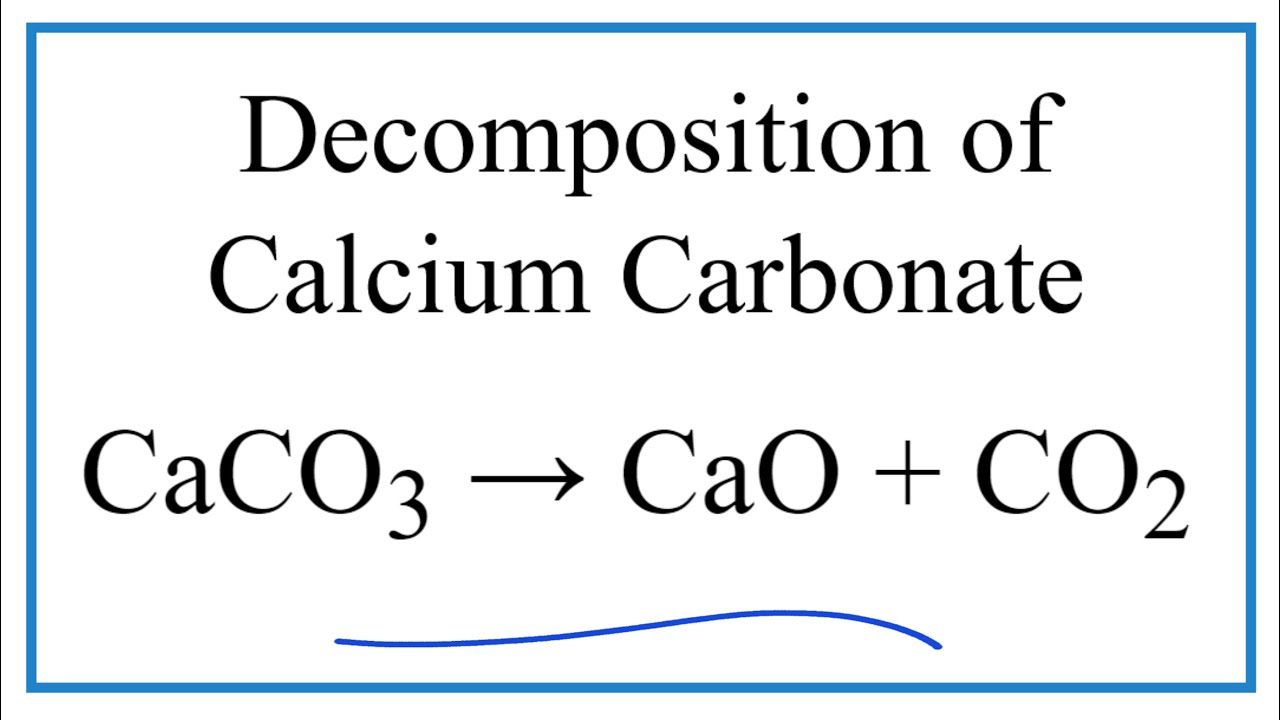
How To Balance Caco3 Cao Co2 Decomposition Of Calcium Carbonate With Heat Youtube

How To Balance Caco3 Cao Co2 Youtube

Type Of Reaction For Caco3 Cao Co2 Youtube

Calcium Carbonate Decomposes On Heating To Form Calcium Oxide And Carbon Dioxide When 10 G Of Youtube
0 Comments